

The low levels of silica mean that Hawaiian magma has a low viscosity, which explains why we see lava readily cascading downslope, and being churned up in the air in great fire fountains.Īt the other end of the spectrum we have magma that is formed from ‘felsic’ volcanic rock, which has a much higher silicon content.
It is the silica content that controls the viscosity of the magma, and hence the nature of the volcanism that is seen. Mafic rock contains relatively little silica. Hawaiian magma is ‘basaltic’, and is a prime example of what results from heating ‘mafic’ volcanic rock. However, the orange rivers flowing down the sides of Hawaiian volcanoes are not typical of what may be found elsewhere. Magma (or lava, as it is called when it reaches the surface) is molten rock, and you are probably already familiar with what it looks like. To understand this, we must first look at what effect silica has on magma.
The most common igneous rocks by far are granites and basalts. In some lava lakes, gases come out as invisible, colourless and odourless gas bursts and may kill many people and animals. Other gases that bubble out at the surface include nitrogen, oxygen, argon, carbon dioxide, boron, carbon monoxide, methane, hydrogen, nitric acid, hydrochloric acid, hydrofluoric acid, sulfuric acid, sulfur dioxide and sulfur trioxide. Some 99% of all gases in lavas are water vapour. Many lavas are vesicular (have cavities), indicating former gas bubbles which escaped from within the magma as it erupted at the surface. Conversely, silica-rich melts favour the crystallisation of silicates such as feldspars, micas and quartz (i.e. The silicate structures that develop favour the crystallisation of minerals of the olivine and pyroxene groups (i.e. Many melts are rich in basic oxides such as MgO and FeO and relatively low in silica. Therefore, a water-rich magma is more fluid than a dry magma of the same composition. Rises in temperature and in water content break down the structure of magmas and decrease their viscosity. Although silica-rich magmas have lower temperatures than silica-poor magmas, a high degree structure gives them higher viscosity. The viscosity of a silicate melt (liquid magma) is controlled by its degree of structure, with increased structure in magmas correlating strongly with increased viscosity. For example, syrup is more viscous than water. Viscosity is the resistance of a fluid to motion.
It ranges from 2.4 g/cm3 (for silica-rich lavas) to 2.9 g/cm3 (for silica-poor lavas). The density of chilled magma (volcanic glass) is usually measured. Komatiitic lavas (very rich in magnesium and low in silica) probably erupted at 1300° C - 1400° C and mostly date from the Archean Era when the earth was hotter. Less silica-rich lavas generally come out at 1000° C - 1230° C while silica-rich lavas are cooler at 750° C - 900° C. The temperature of some lavas can be measured in the field using a thermocouple providing the eruption is not violent (as in lava flows or lakes). for air temperature, distance, and elevation). It can be done at a distance using an optical pyrometer (glowing filament) but this needs many corrections (e.g. The temperature of a magma is hard to measure. The properties of magmas include temperature, density, viscosity, gas content and abundance. Some solidify within the earth (plutonic or subvolcanic rocks). Magmas consolidate at the surface as lava flows (volcanic), or fall as lithic (rock), crystal and/or glass fragments (pyroclastic).


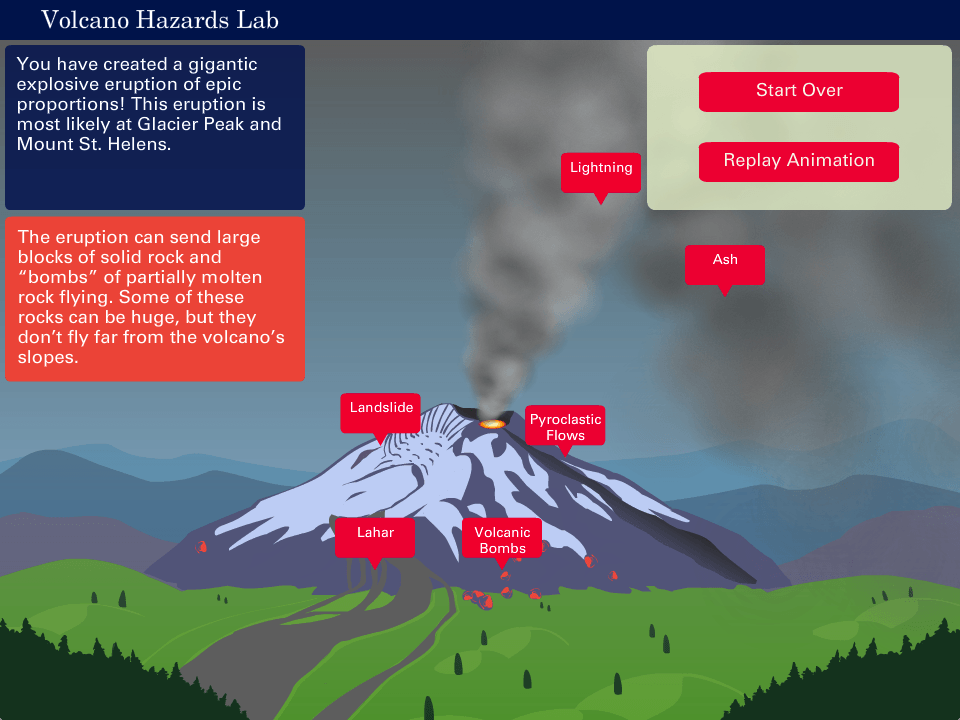
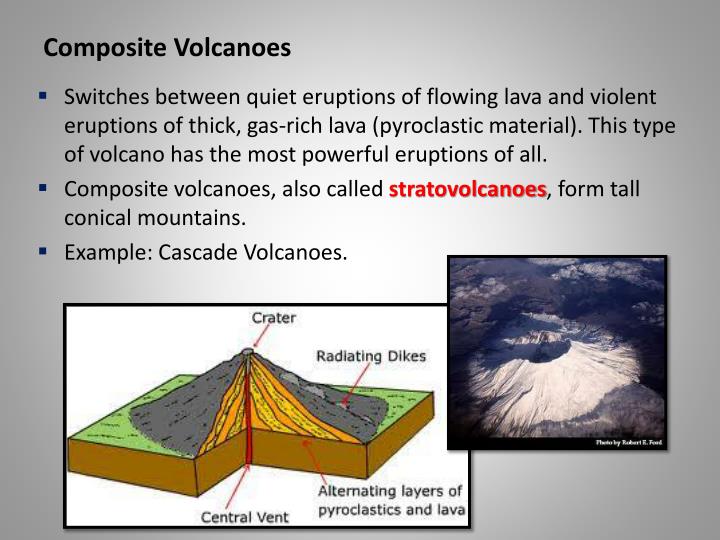
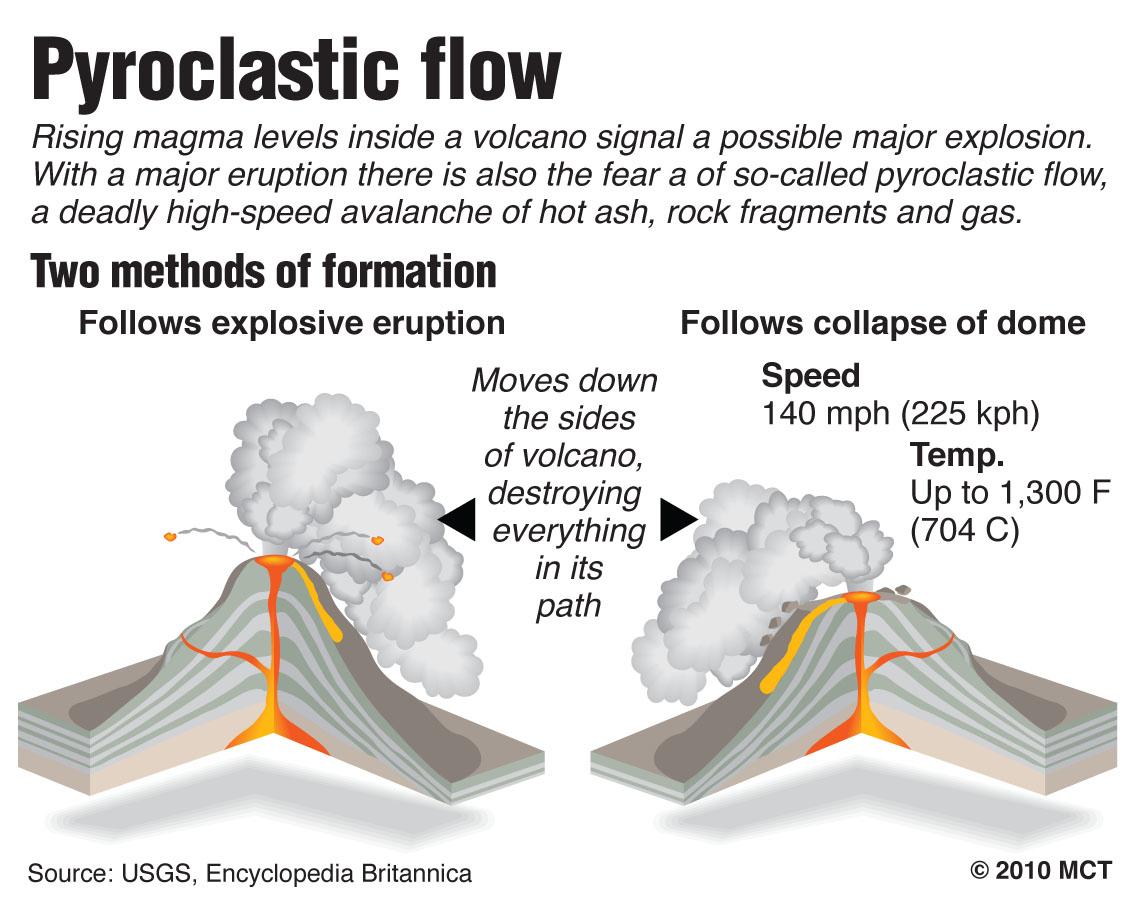
 0 kommentar(er)
0 kommentar(er)
